

A C. diff infection can occur in 2 different ways

Primary
C. diff
infection (pCDI)
is when an individual tests positive for
C. diff
infection, without a recent
positive test (within 8 weeks)5
6 to 11%
MORTALITY
30-day mortality rates4
Recurrent
C. diff
(rCDI)
is when a
C. diff
infection occurs within
8 weeks of a previous infection6-9
16 to 39%
MORTALITY
Escalation in death rate in the US after each
subsequent
C. diff
infection, annually4
Sepsis |
Toxic
megacolon |
Death
Severe diarrhea
Fever
Abdominal pain
Nausea
The standard of care for
treating primary
C. diff
infection is antibiotics,
which is sufficient for
many patients,10
but …

Hospitalizations |
Hospital days |
Clinical
complications
| Surgery
Health-related
quality of life
Physical and
psychological
impact
Work and
daily life
disruptions
Total healthcare
costs
The typical antibiotics used to treat
primary
C. diff
infection are10:
Vancomycin a glycopeptide antibiotic
Fidaxomicin a macrolide antibiotic
In clinical trials,
antibiotics addressed
acute symptoms
of a
C. diff
infection, but many
patients experienced a
recurrent infection.15
28 to 43%
Patients suffered a recurrence
25 days POSTTREATMENT
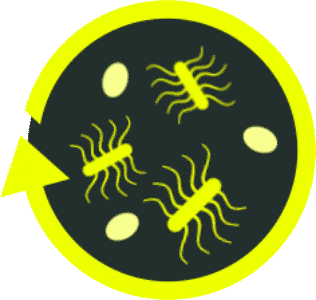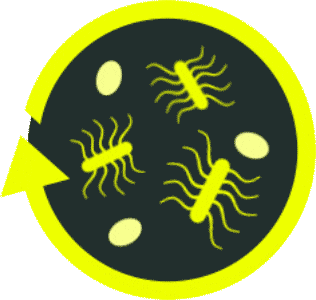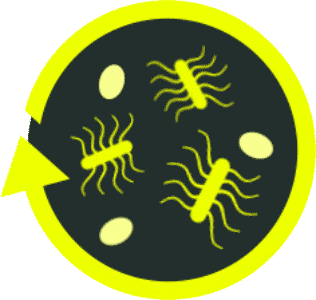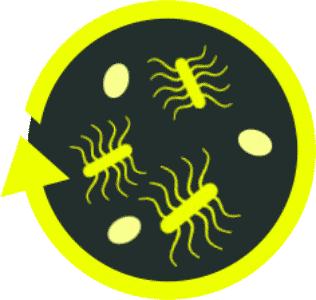
Antibiotics-driven dysbiosis creates an
environment susceptible to recurrence4,9,17,18

Toxin-producing
C. diff
bacteria

C. diff spores

Beneficial
bacteria

C. diff-specific
antibiotics
ABX, antibiotics.

Toxin-producing
C. diff
bacteria

C. diff spores

Beneficial
bacteria

C. diff-specific
antibiotics
ABX, antibiotics.
Antibiotics alone don't address the underlying dysbiosis or the entire
2-phase life cycle of
C. diff, and the risk of recurrence continues with increased frequency and patient impact9,14,18
Antibiotics cannot eliminate the highly resilient C. diff spores, which continue to thrive in the gut without the presence of counterbalancing beneficial gut bacteria.9,18


rCDI presents a unique clinical challenge because of the
paradox of antibiotic treatment on the primary
C. diff
infection, which may lead to continued disruption of the gut
microbiome18
Antibiotic treatment is necessary to address the acute infection, but it can contribute
to the onset of a recurrent
C. diff
infection.











